|
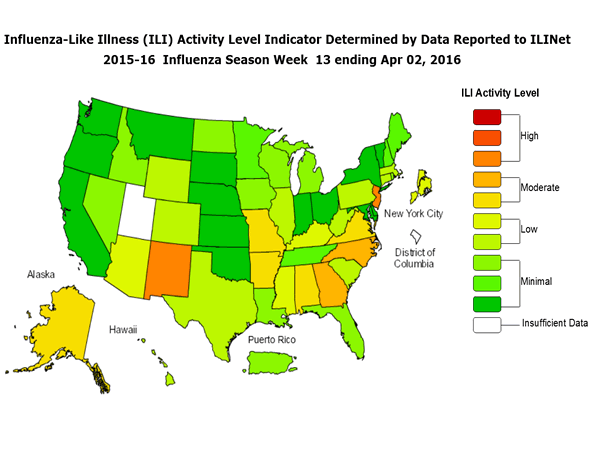
The most recent FluView report shows that flu activity decreased slightly, but remains elevated in United States. While it’s possible that activity might have peaked for the season, some parts of the country are still experiencing high levels of flu activity and ongoing activity is expected to continue for several weeks nationally.
CDC recommends a yearly flu vaccine for everyone 6 months and older. Vaccination can reduce flu illnesses, doctors' visits, and missed work and school due to flu illness, as well as prevent flu-related hospitalizations.
CDC also recommends that patients suspected of having influenza who are at high risk of flu complications or who are very sick with flu-like illness should receive prompt treatment with influenza antiviral drugs without waiting for confirmatory testing.
|