You are subscribed to updates from the Centers for Disease Control and Prevention (CDC).
CDC H1N1 Flu Website Situation Update, February 6, 2010
Key Flu Indicators
Each week CDC analyzes information about influenza disease activity in the United States and publishes findings of key flu indicators in a report called FluView. During the week of January 24-30, 2010, most key flu indicators remained about the same as during the previous week. Below is a summary of the most recent key indicators:
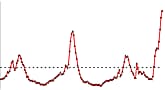
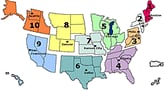
- Visits to doctors for influenza-like illness (ILI) nationally are low. ILI is also looked at by Region. Of 10 regions in the United States, ILI declined or remained about the same, except for in one region of the country. In Region 10 (Arkansas, Oregon, Idaho and Washington), ILI activity increased, but still remains low overall.
- Overall cumulative hospitalization rates for the 2009-10 influenza season have leveled off in all age groups and very few 2009 H1N1-laboratory confirmed hospitalizations were reported by states during the week ending January 30.
- The proportion of deaths attributed to pneumonia and influenza (P&I) based on the 122 Cities Report decreased slightly over the previous week, but is still higher than expected for this time of year. In addition, another nine flu-related pediatric deaths were reported this week: eight of these deaths were associated with laboratory confirmed 2009 H1N1, and one death was associated with an influenza A virus for which the subtype was undetermined. Since April 2009, CDC has received reports of 321 laboratory-confirmed pediatric deaths: 272 due to 2009 H1N1, 47 pediatric deaths that were laboratory confirmed as influenza, but the flu virus subtype was not determined, and two pediatric deaths that were associated with seasonal influenza viruses. (Laboratory-confirmed deaths are thought to represent an undercount of the actual number. CDC has provided estimates about the number of 2009 H1N1 cases and related hospitalizations and deaths.
- No states reported widespread influenza activity. Six states reported regional influenza activity. They are: Alabama, Georgia, Maine, New Jersey, New Mexico and Virginia. Almost all of the influenza viruses identified so far continue to be 2009 H1N1 influenza A viruses. These viruses remain similar to the virus chosen for the 2009 H1N1 vaccine, and remain susceptible to the antiviral drugs oseltamivir and zanamivir with rare exception.
*All data are preliminary and may change as more reports are received.
U.S. Situation Update
U.S. Patient Visits Reported for Influenza-like Illness (ILI)
U.S. Influenza-like Illness (ILI) Reported by Regions
|
Cases Defined by
|
Hospitalizations
|
Deaths
|
|
|---|---|---|---|
| Influenza Laboratory-Tests** | 39,794 | 1,905 | |
|
*Reports can be based on syndromic, admission or discharge data, or a combination of data elements that could include laboratory-confirmed and influenza-like illness hospitalizations. *Laboratory confirmation includes any positive influenza test (rapid influenza tests, RT-PCR, DFA, IFA, or culture), whether or not typing was done. The table shows aggregate reports of all laboratory confirmed influenza hospitalizations and deaths (including 2009 H1N1 and seasonal flu) since August 30, 2009 received by CDC from U.S. states and territories**. This table will be updated weekly each Friday at 11 a.m. For the 2009-2010 influenza season, states are reporting based on new case definitions for hospitalizations and deaths effective August 30, 2009. CDC will continue to use its traditional surveillance systems to track the progress of the 2009-2010 influenza season. For more information about influenza surveillance, including reporting of influenza-associated hospitalizations and deaths, see Questions and Answers: Monitoring Influenza Activity, Including 2009 H1N1. The number of 2009 H1N1 hospitalizations and deaths reported to CDC from April – August 2009 is available on the Past Situation Updates page. For state level information, refer to state health departments. International Human Cases of 2009 H1N1 Flu Infection
**States report weekly to CDC either 1) laboratory-confirmed influenza hospitalizations and deaths or 2) pneumonia and influenza syndrome-based cases of hospitalization and death resulting from all types or subtypes of influenza. Although only the laboratory confirmed cases are included in this report, CDC continues to analyze data both from laboratory confirmed and syndromic hospitalizations and deaths. |
|||
|
Date Reported
|
Laboratory-Confirmed 2009 H1N1 Influenza Pediatric Deaths
|
Laboratory-Confirmed Influenza A Subtype Unknown Pediatric Deaths
|
Laboratory-Confirmed
Seasonal Influenza |
Total |
|---|---|---|---|---|
| This Week (Week 4, January 24 to January 30, 2010) | 8 | 1 | 0 | 9 |
| Since August 30, 2009 | 212 | 44 | 1 | 257 |
| Cumulative since April 26, 2009 | 272 | 47 | 2 | 321 |
|
This table is based on data reported to CDC through the Influenza-Associated Pediatric Mortality Surveillance System. Influenza-associated deaths in children (persons less than 18 years) was added as nationally notifiable condition in 2004. For more information about influenza-associated pediatric mortality, see FluView. |
||||
For more information about the U.S. situation, see the CDC H1N1 Flu U.S. Situation page.
International Situation Update
This report provides an update to the international situation as of January 31, 2010. The World Health Organization (WHO) continues to report laboratory-confirmed 2009 H1N1 flu cases and deaths on its Web page. These laboratory-confirmed cases represent a substantial underestimation of
total cases in the world, as most countries focus surveillance and laboratory testing only on people with severe illness.
The 2009 H1N1 influenza virus continues to be the dominant influenza virus in circulation in the world. For the most recent period in which data are available, from January 17, 2009 to January 23, 2010, 54% were typed as influenza A and 46% as influenza B. Out of all subtyped influenza A viruses, 93% were 2009 H1N1 positive.
In temperate regions of the Southern Hemisphere, sporadic cases of 2009 H1N1 continue to be reported but no substantial increases in influenza activity have been observed. In the northern temperate and tropical regions of the Americas, 2009 H1N1 activity continues to decrease or remain low in most places. Influenza transmission continues to remain active in North Africa, certain areas of Eastern and Southeastern Europe, and parts of South and East Asia
For more information about the international situation, see the CDC H1N1 Flu International Situation page.
Recent Updates of Interest
- UPDATE: Weekly FluView Map and Surveillance Report for Week Ending January 30, 2010
During week 4 (January 24-30, 2010), influenza activity remained at approximately the same levels as last week in the U.S.119 (3.2%) specimens tested by U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories and reported to CDC/Influenza Division were positive for influenza. - UPDATE: Influenza and Pneumonia-Associated Hospitalizations and Deaths from August 30, 2009 to January 30, 2010
FluView reports that for the week of January 24-30, 2010, flu activity in the United States remained about the same as during the previous week. Flu activity is relatively low at this time, with most flu continuing to be caused by 2009 H1N1. Flu activity, caused by either 2009 H1N1 or seasonal flu viruses, may rise and fall, but it is expected to continue for several more months. - NEW: Shortened Expiration Period For Sanofi Pasteur 2009 H1N1 Vaccine In Pre-filled Syringes Questions & Answers
All lots of monovalent 2009 H1N1 influenza vaccine in pre-filled syringes manufactured by Sanofi Pasteur, not included in the two earlier recalls, should now be administered by February 15, 2010 regardless of the expiration imprinted on the package. - NEW: Non-Safety-Related Voluntary Recall Of Sanofi Pasteur 2009 H1N1 Flu Vaccine In Pre-filled Syringes Questions and Answers
In recent testing of its influenza A (H1N1) monovalent vaccine, Sanofi Pasteur found five distributed lots of single-dose, pre-filled syringe pediatric (0.25 mL) vaccine and one distributed lot of single-dose pre-filled syringe for older children and adults (0.5 mL) vaccine had potency below pre-specified limits. - CDC Health Alert Network (HAN) Info Service Message: Non-Safety-Related Voluntary Recall of Unused Doses from Certain Lots of Sanofi Pasteur H1N1 Vaccine in Pre-Filled Syringes
As of January 7, 2010, the cumulative pro rata allocation is approximately 136 million doses of 2009 H1N1 vaccine. As of January 5, 2010, approximately 111 million doses have been shipped, so supplies of 2009 H1N1 vaccine available to be administered are ample. Although the 2009 H1N1 vaccine was initially prioritized to certain target groups, due to the increase in supply most jurisdictions are now making vaccine available for everyone who wishes to receive it.
Additional Updates on the CDC H1N1 Flu Website
To learn about other recent updates made to the CDC H1N1 Flu Website, please check the "What's New" page on the CDC H1N1 Flu website.
Get H1N1 Updates & Health Tips via Text Message
 Sign up to get health updates sent via text message. Messages are sent about three times a week with relevant H1N1 flu updates and timely
health tips.
Sign up to get health updates sent via text message. Messages are sent about three times a week with relevant H1N1 flu updates and timely
health tips.
Text UPDATES to 87000 to sign up.
To learn more, see www.cdc.gov/mobile.
Modify/Update Subscriber Preferences | Unsubscribe | Send Feedback | Learn more about CDC Email Updates
To receive the latest news for your region, please update your profile with your country, state and zip code.
Questions or problems? Please contact support@xxxxxxxxxxxxxxx.

|
Fight Flu with Facts! • Visit Flu.gov
|

|
Centers for Disease Control and Prevention (CDC) · 1600 Clifton Rd · Atlanta GA 30333 · 800-CDC-INFO (800-232-4636)


