You are subscribed to updates from the Centers for Disease Control and Prevention (CDC).
Key Flu Indicators
Each week CDC analyzes information about influenza disease activity in the United States and publishes findings of key flu indicators in a report called FluView.* During the week of November 1-7, 2009, a review of key indicators found that certain indicators declined, while others continued to rise. Overall, flu activity in the United Sates remained very high. Below is a summary of the most recent key indicators:
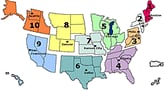
- Visits to doctors for influenza-like illness (ILI) nationally decreased this week over last week. This is the second week of national decreases in ILI after four consecutive weeks of sharp increases. (All regions but one showed declines in ILI. Region I (CT, ME, MA, NH, RI and VT) continues to show sharp increases in ILI activity. While ILI declined overall nationally, visits to doctors for influenza-like illness remain higher than what is seen during the peak of many regular flu seasons.
- Total influenza hospitalization rates for laboratory-confirmed flu continue to climb and remain higher than expected for this time of year. Hospitalization rates continue to be highest in younger populations with the highest hospitalization rate reported in children 0-4 years old.
- The proportion of deaths attributed to pneumonia and influenza (P&I) based on the 122 Cities Report continues to increase and has been higher than what is expected for six weeks now. In addition, 35 flu-related pediatric deaths were reported this week: 26 of these deaths were associated with laboratory confirmed 2009 H1N1; eight were influenza A viruses, but were not subtyped; and one was an influenza B virus. Since April 2009, CDC has received reports of 156 laboratory-confirmed pediatric 2009 H1N1 deaths, one influenza B death, and another 23 pediatric deaths that were laboratory confirmed as influenza, but the flu virus subtype was not determined.
- Forty-six states are reporting widespread influenza activity at this time; a decline of two states over last week. They are: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming. This many reports of widespread activity at this time of year are unprecedented during seasonal flu.
- Almost all of the influenza viruses identified so far continue to be 2009 H1N1 influenza A viruses. These viruses remain similar to the virus chosen for the 2009 H1N1 vaccine, and remain susceptible to the antiviral drugs oseltamivir and zanamivir with rare exception
*All data are preliminary and may change as more reports are received.
U.S. Situation Update
U.S. Patient Visits Reported for Influenza-like Illness (ILI)
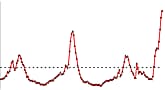
U.S. Influenza-like Illness (ILI) Reported by Regions
|
Cases Defined by
|
Hospitalizations
|
Deaths
|
|
|---|---|---|---|
| Influenza Laboratory-Tests** | 22,364 | 877 | |
|
*Reports can be based on syndromic, admission or discharge data, or a combination of data elements that could include laboratory-confirmed and influenza-like illness hospitalizations. *Laboratory confirmation includes any positive influenza test (rapid influenza tests, RT-PCR, DFA, IFA, or culture), whether or not typing was done. The table shows aggregate reports of all laboratory confirmed influenza hospitalizations and deaths (including 2009 H1N1 and seasonal flu) since August 30, 2009 received by CDC from U.S. states and territories**. This table will be updated weekly each Friday at 11 a.m. For the 2009-2010 influenza season, states are reporting based on new case definitions for hospitalizations and deaths effective August 30, 2009. CDC will continue to use its traditional surveillance systems to track the progress of the 2009-2010 influenza season. For more information about influenza surveillance, including reporting of influenza-associated hospitalizations and deaths, see Questions and Answers: Monitoring Influenza Activity, Including 2009 H1N1. The number of 2009 H1N1 hospitalizations and deaths reported to CDC from April – August 2009 is available on the Past Situation Updates page. For state level information, refer to state health departments. International Human Cases of 2009 H1N1 Flu Infection
**States report weekly to CDC either 1) laboratory-confirmed influenza hospitalizations and deaths or 2) pneumonia and influenza syndrome-based cases of hospitalization and death resulting from all types or subtypes of influenza. Although only the laboratory confirmed cases are included in this report, CDC continues to analyze data both from laboratory confirmed and syndromic hospitalizations and deaths. |
|||
|
Date Reported
|
Laboratory-Confirmed 2009 H1N1 Influenza Pediatric Deaths
|
Laboratory-Confirmed Influenza A Subtype Unknown Pediatric Deaths
|
Laboratory-Confirmed
Seasonal Influenza |
Total |
|---|---|---|---|---|
| This Week (Week 44, November 1-7, 2009) | 26 | 8 | 1 | 35 |
| Since August 30, 2009 | 98 | 19 | 0 | 117 |
| Cumulative since April 26, 2009 | 156 | 22 | 1 | 179 |
|
This table is based on data reported to CDC through the Influenza-Associated Pediatric Mortality Surveillance System. Influenza-associated deaths in children (persons less than 18 years) was added as nationally notifiable condition in 2004. For more information about influenza-associated pediatric mortality, see FluView. |
||||
For more information about the U.S. situation, see the CDC H1N1 Flu U.S. Situation page.
International Situation Update
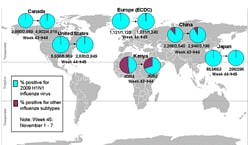 This report provides an update to the international situation as of November 13, 2009. The World Health Organization (WHO) continues to report laboratory-confirmed 2009 H1N1 flu cases and deaths on its Web page. These laboratory-confirmed cases represent a substantial underestimation of total cases in the
world, as many countries focus surveillance and laboratory testing only on people with severe illness. The 2009 H1N1 influenza virus continues to be the dominant influenza virus in circulation in the world. Since April 19, 2009, more than 65% of all influenza positive specimens reported to WHO have been 2009 H1N1. In temperate regions of the Southern Hemisphere, little disease activity due to 2009 H1N1 has been reported. In tropical regions of the Americas and Asia, influenza activity due to
2009 H1N1 remains variable. In temperate regions of the Northern Hemisphere, influenza-like illness (ILI) activity due to 2009 H1N1 continues to increase across many countries in Europe and Asia, as well as many areas of the United States, Mexico and Canada.
This report provides an update to the international situation as of November 13, 2009. The World Health Organization (WHO) continues to report laboratory-confirmed 2009 H1N1 flu cases and deaths on its Web page. These laboratory-confirmed cases represent a substantial underestimation of total cases in the
world, as many countries focus surveillance and laboratory testing only on people with severe illness. The 2009 H1N1 influenza virus continues to be the dominant influenza virus in circulation in the world. Since April 19, 2009, more than 65% of all influenza positive specimens reported to WHO have been 2009 H1N1. In temperate regions of the Southern Hemisphere, little disease activity due to 2009 H1N1 has been reported. In tropical regions of the Americas and Asia, influenza activity due to
2009 H1N1 remains variable. In temperate regions of the Northern Hemisphere, influenza-like illness (ILI) activity due to 2009 H1N1 continues to increase across many countries in Europe and Asia, as well as many areas of the United States, Mexico and Canada.
For more information about the international situation, see the CDC H1N1 Flu International Situation page.
CDC Releases New Widget: 5 in 5: Clinician Quick Facts for 2009 H1N1
 CDC released a new widget this week for clinicians. The "5 in 5: Clinician Quick Facts for 2009 H1N1" widget provides five quick facts for health care providers and clinicians to consider when evaluating a patient for antiviral treatment. This and other CDC
widgets are available at http://www.cdc.gov/widgets.
CDC released a new widget this week for clinicians. The "5 in 5: Clinician Quick Facts for 2009 H1N1" widget provides five quick facts for health care providers and clinicians to consider when evaluating a patient for antiviral treatment. This and other CDC
widgets are available at http://www.cdc.gov/widgets.
CDC Releases New Video: H1N1 Flu Vaccine – Why the Delay?
 Flu vaccine is the single best way to protect against influenza illness. Watch a short video to understand how flu vaccines are made, why manufacturing and shipping vaccine take so
long, and how you can find flu vaccines near you.
Flu vaccine is the single best way to protect against influenza illness. Watch a short video to understand how flu vaccines are made, why manufacturing and shipping vaccine take so
long, and how you can find flu vaccines near you.
You can learn more about flu vaccines at www.cdc.gov/h1n1flu or www.cdc.gov/flu.
To view and download links for other CDC videos, visit CDC-TV www.cdc.gov/CDCTV or CDC's YouTube channel www.youtube.com/cdcstreaminghealth.
Recent Updates of Interest
- 2009 H1N1 Influenza Vaccine Supply Status
Every Friday, CDC will post updated state-by-state 2009 H1N1 vaccine supply and distribution data. 26,248,100 doses have been shipped as of November 11, 2009. - Weekly FluView Map and Surveillance Report for Week Ending November 7, 2009
During the week of November 1-7, 2009, influenza activity remained high in the United States as reported in FluView. Flu activity is widespread in 48 states. Nationally, visits to doctors for influenza-like-illness declined slightly from last week, but are still very high. Flu-related hospitalizations and deaths continue to increase and are very high nation-wide compared to what is expected for this time of year. - 2009 H1N1 Flu: International Situation Update
This report provides an update to the international situation as of November 13, 2009. The World Health Organization (WHO) continues to report updated 2009 H1N1 flu-associated laboratory-confirmed cases and deaths on its Web page. - U.S. Influenza and Pneumonia-Associated Hospitalizations and Deaths from August 30 to November 7, 2009.
During the week of November 1-7, 2009, influenza activity continued to increase in the United States as reported in FluView. Flu activity is now widespread. Nationwide, visits to doctors for influenza-like-illness are increasing steeply and are now higher than what is seen at the peak of many regular flu seasons. In addition, flu-related hospitalizations and deaths continue to go up nation-wide and are above what is expected for this time of year. - Information for Pregnant Women Working in Education, Child Care, and Health Care Settings
This revised document updates the information for employed women so that it is consistent with the most recent infection control guidance posted by CDC. - NEW: CDC Estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April - October 17, 2009
CDC has developed a method to provide an estimated range of the total number of 2009 H1N1 cases, hospitalizations and deaths in the United States since April, 2009, as well as a breakdown of these estimates by age groups... - New: 2009 H1N1 and People with Diabetes
Influenza related information for people with diabetes - Update: Frequently asked questions on use of influenza A(H1N1) 2009 monovalent vaccines (2009 H1N1 monovalent influenza vaccines): Practical considerations for immunization programs and providers
Two different influenza vaccines are available this influenza season, and many people will be recommended to receive both the seasonal influenza vaccine and the 2009 influenza A (H1N1) 2009 monovalent vaccine (referred to in this document as 2009 H1N1 monovalent influenza vaccine). Below are some practical considerations for use of influenza vaccines. This information is only intended to address the current flu season and might change as the situation unfolds. This information is not intended to be applied to routine use during future seasonal influenza vaccination efforts. - Interim Guidance: Considerations Regarding 2009 H1N1 Influenza in Intrapartum and Postpartum Hospital Settings
This updated guidance replaces previously posted guidance entitled "Consideration Regarding Novel H1N1 Flu Virus in Obstetric Setting", dated July 6, 2009. Two steps are provided to guide the clinical management of labor, delivery and postpartum care of a mother with suspected or confirmed maternal infection with 2009 H1N1 flu, and care of the newborn. - Letter to Providers Promoting PPSV for Adults
Pneumococcal infections have been identified as an important complication in severe and fatal cases of 2009 H1N1 influenza virus infection. CDC has written a letter to providers urging them to make sure all their adult patients with indications have received the pneumococcal polysaccharide vaccine.
Additional Updates on the CDC H1N1 Flu Website
To learn about other recent updates made to the CDC H1N1 Flu Website, please check the "What's New" page on the CDC H1N1 Flu website.
Modify/Update Subscriber Preferences | Unsubscribe | Send Feedback | Learn more about CDC Email Updates
To receive the latest news for your region, please update your profile with your country, state and zip code.
Questions or problems? Please contact support@xxxxxxxxxxxxxxx.

|
Fight Flu with Facts! • Visit Flu.gov
|

|
Centers for Disease Control and Prevention (CDC) · 1600 Clifton Rd · Atlanta GA 30333 · 800-CDC-INFO (800-232-4636)

