You are subscribed to updates from the Centers for Disease Control and Prevention (CDC).
Key Flu Indicators
Each week CDC analyzes information about influenza disease activity in the United States and publishes findings of key flu indicators in a report called FluView. During the week of September 6-12, 2009, a review of the key indictors found that influenza activity continued to increase in the United States compared to the prior weeks. Below is a summary of the most recent key indicators:
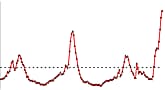
- Visits to doctors for influenza-like illness (ILI) are increasing nationally. Visits to doctors for influenza-like illness are higher than what is expected during this time of year and have increased for five consecutive weeks now. This is very unusual for this time of year.
- Total influenza hospitalization rates for adults and children are similar to or lower than seasonal influenza hospitalization rates depending on age group, but are higher than expected for this time of year.
- The proportion of deaths attributed to pneumonia and influenza (P&I) was low and within the bounds of what is expected at this time of year.
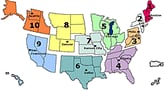
- Twenty-one states are reporting widespread influenza activity at this time. They are: Alabama, Alaska, Arizona, Arkansas, Florida, Georgia, Illinois, Kansas, Kentucky, Louisiana, Maryland, Minnesota, Mississippi, Nevada, New Mexico, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, and Virginia. Any reports of widespread influenza activity in September are very unusual.
- Almost all of the influenza viruses identified so far are 2009 H1N1 influenza A viruses. These viruses remain similar to the viruses chosen for the 2009 H1N1 vaccine, and remain susceptible to the antiviral drugs oseltamivir and zanamivir with rare exceptions.
U.S. Situation Update
U.S. Patient Visits Reported for Influenza-like Illness (ILI)
U.S. Influenza-like Illness (ILI) Reported by Regions
|
Cases Defined by
|
Hospitalizations
|
Deaths
|
|
|---|---|---|---|
| Influenza and Pneumonia Syndrome* | 3,534 | 291 | |
| Influenza Laboratory-Tests** | 1,035 | 73 | |
| Totals: | 4,569 | 364 | |
|
*Reports can be based on syndromic, admission or discharge data, or a combination of data elements that could include laboratory-confirmed and influenza-like illness hospitalizations. **Laboratory confirmation includes any positive influenza test (rapid influenza tests, RT-PCR, DFA, IFA, or culture), whether or not typing was done. This table is based on data from a new influenza and pneumonia hospitalizations and deaths web-based reporting system that will be used to monitor trends in activity. This is the second week of data from this new system and reflects reports by 39 of 56 jurisdictions this week. The table shows aggregate reports of all influenza and pneumonia-associated hospitalizations and deaths (including 2009 H1N1 and seasonal flu) since August 30, 2009 received by CDC from U.S. states and territories. This table will be updated weekly each Friday at 11 a.m. For the 2009-2010 influenza season, states are reporting based on new case definitions for hospitalizations and deaths effective August 30, 2009. CDC will continue to use its traditional surveillance systems to track the progress of the 2009-2010 influenza season. For more information about influenza surveillance, including reporting of influenza-associated hospitalizations and deaths, see Questions and Answers: Monitoring Influenza Activity, Including 2009 H1N1. The number of 2009 H1N1 hospitalizations and deaths reported to CDC from April – August 2009 is available on the Past Situation Updates page. For state level information, refer to state health departments. International Human Cases of 2009 H1N1 Flu Infection
|
|||
For more information about the U.S. situation, see the 2009 H1N1 Flu U.S. Situation Update page
.International Situation Update
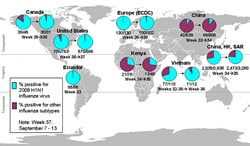
This report provides an update to the international situation as of September 18, 2009. As of September 13, 2009, the World Health Organization (WHO) regions have reported more than 296,471 laboratory-confirmed cases of 2009 H1N1 influenza virus (2009 H1N1) with at least 3,486 deaths, which is an increase of at least 18,864 cases and 281 deaths since September 6th. The laboratory-confirmed cases represent a substantial underestimation of total cases in the world, as many countries focus surveillance and laboratory testing only on people with severe illness. The 2009 H1N1 influenza virus continues to be the dominant influenza virus in circulation in the world. Since April 2009, 60.7% of influenza specimens reported to WHO were 2009 H1N1 viruses. In temperate regions of the Southern Hemisphere, disease due to 2009 H1N1 is largely declining. In tropical regions, there is still substantial disease due to 2009 H1N1. In temperate regions of the Northern Hemisphere, there is some increased disease activity due to 2009 H1N1, including in parts of the United States, Canada and Europe.
For more information about the U.S. situation, see the 2009 H1N1 Flu International Situation Update page.
Recent Updates of Interest
- Weekly FluView Map and Surveillance Report for Week Ending September 12, 2009
During week 36 (September 6-12, 2009), influenza activity remained stable in the United States; however, there were still higher levels of influenza-like illness than is normal for this time of year. - UPDATED What To Do If You Get Sick: 2009 H1N1 and Seasonal Flu
How do I know if I have the flu? What should I do if I get sick? What are the emergency warning signs? More... - UPDATED CDC Novel H1N1 Vaccination Planning Q&A
When will the decision to administer vaccine be made? When will vaccine shipping begin? How many manufacturers are producing vaccine? More... - UPDATE 2009 H1N1 Influenza Vaccine and Pregnant Women
Questions and Answers about 2009 H1N1 Influenza Vaccine and Pregnant Women - U.S. Influenza and Pneumonia-Associated Hospitalizations and Deaths from September 6-12, 2009
As of 11:00 AM ET on September 18, 2009, CDC is reporting 4,569 hospitalizations and 364 deaths. This is the second week of data from this new system and reflects reports by 39 of 56 jurisdictions this week. This reports all influenza and pneumonia-associated hospitalizations and deaths (including 2009 H1N1 and seasonal flu) since August 30, 2009 received by CDC from U.S. states and territories. - 2009 H1N1 Flu: International Situation Update
This report provides an update to the international situation as of September 18, 2009. As of September 13, 2009, the World Health Organization (WHO) regions have reported more than 296,471 laboratory-confirmed cases of 2009 H1N1 influenza virus (2009 H1N1) with at least 3,486 deaths. - Updated Interim Recommendations for Obstetric Health Care Providers Related to Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009-2010 Season
- Planning for 2009 H1N1 Influenza: A Preparedness Guide for Small Business
Small businesses play a key role in protecting employees' health and safety as well as limiting the impact to the economy and society during an influenza pandemic. Advance planning for pandemic influenza, a novel infectious disease that could occur in varying levels of severity, is critical. Companies that provide critical services, such as power and telecommunications, have a special responsibility to their community to plan for continued operations in a pandemic and should plan accordingly. - Asthma Information for Patients and Parents of Patients
Anyone with asthma is at higher risk for flu-related complications, such as pneumonia. - General Questions and Answers on Guillain-Barre syndrome (GBS)
What is GBS? What causes GBS? Who is at risk for developing GBS? Do vaccines cause GBS? How common is GBS, and how common is it after people are vaccinated for seasonal influenza? What happened in 1976 with GBS and the swine flu vaccine? Why did some people develop GBS after they received the 1976 swine flu vaccine? More... - General Questions and Answers on 2009 H1N1 Influenza A Vaccine Safety
Will the 2009 H1N1 influenza vaccines be safe? Are there any side effects to the 2009 H1N1 influenza vaccine? Are there some people who should not receive this vaccine? How will the 2009 H1N1 influenza vaccines be monitored for safety? Will the 2009 H1N1 vaccines that are currently recommended contain adjuvants? More... - General Questions and Answers on Thimerosal
What is thimerosal? What are preservatives and why are they used in vaccines? Will the 2009 H1N1 influenza vaccine contain thimerosal? I have concerns about the use of thimerosal. Is thimerosal still being used? Is thimerosal safe when used as a preservative in vaccines? - 2009 Influenza (H1N1) monovalent vaccine: Vaccine Provider Agreement Q&A
The purpose of this document is to answer questions pertaining to the 2009 Influenza (H1N1) monovalent vaccine Vaccine Provider Agreement. The provider agreement is an agreement between providers and public health at the Project Area level and indicates the minimum federal requirements for providers to participate in the H1N1 influenza vaccination effort. - School-Located Vaccination Planning Materials and Templates
These documents were designed to provide information for planning and conducting school-located 2009 H1N1 influenza vaccination clinics that target school-aged children enrolled in school and potentially other groups in the community. The targeted audience for these materials is primarily state and local public health department immunization and preparedness staff who are responsible for carrying out 2009 H1N1 influenza vaccination, but also education officials, school nurses, and others who are interested in planning and carrying out such activities.
Additional Updates on the CDC H1N1 Flu Website
To learn about other recent updates made to the CDC H1N1 Flu Website, please check the "What's New" page on the CDC H1N1 Flu website.
Modify/Update Email Preferences | Unsubscribe | Send Feedback | Learn more about CDC Email Updates
To receive the latest news for your region, please update your profile with your country, state and zip code.
Questions or problems? Please contact support@xxxxxxxxxxxxxxx.


|
|
|
Centers for Disease Control and Prevention (CDC) · 1600 Clifton Rd · Atlanta GA 30333 · 800-CDC-INFO (800-232-4636)