You are subscribed to updates from the Centers for Disease Control and Prevention (CDC).
Vaccination Recommendations
With the new H1N1 virus continuing to cause illness, hospitalizations and deaths in the US during the normally flu-free summer months and some uncertainty and about what the upcoming flu season might bring, CDC's Advisory Committee on Immunization Practices has taken an important step in preparations for a voluntary novel H1N1 vaccination effort to counter a possibly severe upcoming flu season. On July 29, ACIP met to consider who should receive novel H1N1 vaccine when it becomes available.
U.S. Situation Update
U.S. Patient Visits Reported for Influenza-like Illness (ILI)
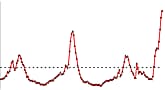
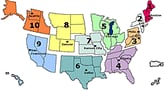
U.S. Influenza-like Illness (ILI) Reported by Regions
|
Reporting States and Territories*
|
Hospitalized Cases
|
Deaths
|
|
|---|---|---|---|
| 47 | 5,514 | 353 | |
|
*Includes the District of Columbia, American Samoa, Guam, Puerto Rico and the U.S. Virgin Islands. The number of hospitalized novel H1N1 cases and deaths presented in this table are an aggregate of reports received by CDC from U.S. states and territories and will be updated weekly each Friday at 11am. For state level information, refer to state health departments. CDC discontinued reporting of individual confirmed and probable cases of novel H1N1 infection on July 24, 2009. CDC will report the total number of hospitalizations and deaths weekly, and continue to use its traditional surveillance systems to track the progress of the novel H1N1 flu outbreak.
International Human Cases of H1N1 Flu Infection
|
|||
For more information about the U.S. situation, see the CDC H1N1 Flu website.
International Situation Update
 This situation report provides an update of the international situation as of July 28, 2009. As of July 27, WHO regions have reported 134,503 laboratory-confirmed cases of novel influenza A (H1N1) and 816 deaths. The lab-confirmed cases represent an underestimation of total cases in the world as many countries have shifted to strategies
of clinical confirmation and prioritization of laboratory testing for only persons with severe illness and/or high risk conditions. Currently, the novel influenza A (H1N1) virus is the dominant influenza virus in circulation in New Zealand, Australia, Chile, Argentina and Brazil. Influenza viruses currently being detected in England and Canada are almost exclusively novel Influenza A (H1N1). Following a seasonal influenza season that was dominated by influenza A (H3N2) virus circulation, South
Africa is now detecting novel influenza A (H1N1) through its routine surveillance system. However, currently influenza A (H3N2) remains the dominant virus in circulation in South Africa. Many seasonal influenza viruses from these countries have not been subtyped. Of those that have been subtyped in Australia, South Africa, and Argentina, the majority are influenza A (H3N2) viruses.This situation report provides an update of the international situation as of July 28, 2009. As of July 27, WHO
regions have reported 134,503 laboratory-confirmed cases of novel influenza A (H1N1) and 816 deaths. The lab-confirmed cases represent an underestimation of total cases in the world as many countries have shifted to strategies of clinical confirmation and prioritization of laboratory testing for only persons with severe illness and/or high risk conditions. Currently, the novel influenza A (H1N1) virus is the dominant influenza virus in circulation in New Zealand, Australia, Chile, Argentina and
Brazil. Influenza viruses currently being detected in England and Canada are almost exclusively novel Influenza A (H1N1). Following a seasonal influenza season that was dominated by influenza A (H3N2) virus circulation, South Africa is now detecting novel influenza A (H1N1) through its routine surveillance system. However, currently influenza A (H3N2) remains the dominant virus in circulation in South Africa. Many seasonal influenza viruses from these countries have not been subtyped. Of those
that have been subtyped in Australia, South Africa, and Argentina, the majority are influenza A (H3N2) viruses.
This situation report provides an update of the international situation as of July 28, 2009. As of July 27, WHO regions have reported 134,503 laboratory-confirmed cases of novel influenza A (H1N1) and 816 deaths. The lab-confirmed cases represent an underestimation of total cases in the world as many countries have shifted to strategies
of clinical confirmation and prioritization of laboratory testing for only persons with severe illness and/or high risk conditions. Currently, the novel influenza A (H1N1) virus is the dominant influenza virus in circulation in New Zealand, Australia, Chile, Argentina and Brazil. Influenza viruses currently being detected in England and Canada are almost exclusively novel Influenza A (H1N1). Following a seasonal influenza season that was dominated by influenza A (H3N2) virus circulation, South
Africa is now detecting novel influenza A (H1N1) through its routine surveillance system. However, currently influenza A (H3N2) remains the dominant virus in circulation in South Africa. Many seasonal influenza viruses from these countries have not been subtyped. Of those that have been subtyped in Australia, South Africa, and Argentina, the majority are influenza A (H3N2) viruses.This situation report provides an update of the international situation as of July 28, 2009. As of July 27, WHO
regions have reported 134,503 laboratory-confirmed cases of novel influenza A (H1N1) and 816 deaths. The lab-confirmed cases represent an underestimation of total cases in the world as many countries have shifted to strategies of clinical confirmation and prioritization of laboratory testing for only persons with severe illness and/or high risk conditions. Currently, the novel influenza A (H1N1) virus is the dominant influenza virus in circulation in New Zealand, Australia, Chile, Argentina and
Brazil. Influenza viruses currently being detected in England and Canada are almost exclusively novel Influenza A (H1N1). Following a seasonal influenza season that was dominated by influenza A (H3N2) virus circulation, South Africa is now detecting novel influenza A (H1N1) through its routine surveillance system. However, currently influenza A (H3N2) remains the dominant virus in circulation in South Africa. Many seasonal influenza viruses from these countries have not been subtyped. Of those
that have been subtyped in Australia, South Africa, and Argentina, the majority are influenza A (H3N2) viruses.
Recent Updates of Interest
- Weekly FluView Map and Surveillance Report for Week Ending July 25, 2009
During week 29 (July 19-25, 2009), influenza activity decreased in the United States; however, there were still higher levels of influenza-like illness than is normal for this time of year. - Novel H1N1 Flu: Facts and Figures
When the novel H1N1 flu outbreak was first detected in mid-April 2009, CDC began working with states to collect, compile and analyze information regarding the novel H1N1 outbreak. On July 24, 2009 official reporting of individual cases of confirmed and probable novel H1N1 infection was discontinued. This page provides a summary of information gathered during the first weeks of the outbreak. These key disease characteristics are thought to remain an accurate representation of novel H1N1 flu. - Novel H1N1 Vaccination Recommendations
With the new H1N1 virus continuing to cause illness, hospitalizations and deaths in the US during the normally flu-free summer months and some uncertainty and about what the upcoming flu season might bring, CDC's Advisory Committee on Immunization Practices has taken an important step in preparations for a voluntary novel H1N1 vaccination effort to counter a possibly severe upcoming flu season. On July 29, ACIP met to consider who should receive novel H1N1 vaccine when it becomes available. - Managing Calls and Call Centers during a Large-Scale Influenza Outbreak: Implementation Tool
During a response to a large-scale influenza outbreak such as the current H1N1 outbreak, a community�s 9-1-1 and healthcare systems may experience a surge in calls or walk-in visits for care, advice, and information. In fact, call volumes or walk-in visits could reach the point of overwhelming the 9-1-1 and healthcare systems, rendering them unable to respond to other emergencies in an efficient and effective manner. - Press Release: CDC Advisors Make Recommendations for Use of Vaccine Against Novel H1N1
The CDC's Advisory Committee on Immunization Practices (ACIP) met July 29, 2009, to develop recommendations on who should receive vaccine against novel influenza A (H1N1) when it becomes available, and to determine which groups of the population should be prioritized if the vaccine is initially available in extremely limited quantities. The committee recommended the vaccination efforts focus on five key populations. - Audio and Transcript for July 29 CDC Press Briefing
CDC Press Conference on Recommendations for Use of Vaccine Against Novel Influenza A (H1N1) - Interim Guidance for the Detection of Novel Influenza A Virus Using Rapid Influenza Diagnostic Tests
This interim guidance provides an overview of the sensitivities of rapid influenza diagnostic tests (RIDT) in detecting novel influenza A (H1N1) virus in order to help guide the reporting and interpretation of test results. - Updated: Questions & Answers Novel H1N1 Influenza Vaccine
Includes Questions & Answers such as "Will vaccination against the new H1N1 influenza be mandatory?" - Novel H1N1 Vaccination Guidance for State, Local, Tribal and Territorial Health Officials
New page launched to provide resources for state and local governments. Includes General Planning Information, Large Scale Vaccination Clinic Planning, and Vaccine Storage and Handling. - Weekly FluView Map and Surveillance Report for Week Ending July 18, 2009
During week 28 (July 12-18, 2009), influenza activity decreased in the United States, however, there were still higher levels of influenza-like illness than is normal for this time of year.
Additional Updates on the CDC H1N1 Flu Website
To learn about other recent updates made to the CDC H1N1 Flu Website, please check the "What's New" page on the CDC H1N1 Flu website
Modify/Update Email Preferences | Unsubscribe | Send Feedback | Learn more about CDC Email Updates
To receive the latest news for your region, please update your profile with your country, state and zip code.
Questions or problems? Please contact support@xxxxxxxxxxxxxxx.


|
|
|
Centers for Disease Control and Prevention (CDC) · 1600 Clifton Rd · Atlanta GA 30333 · 800-CDC-INFO (800-232-4636)

